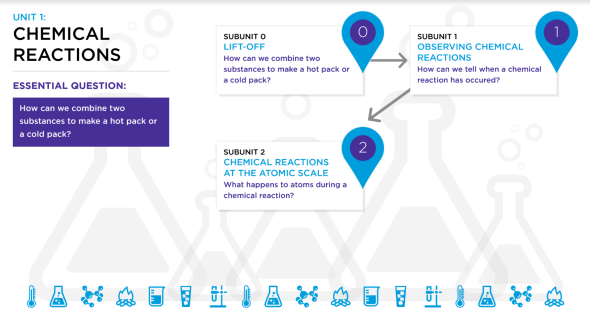
7th Grade Unit 1: Chemical Reactions Roadmap
After Unit 0: Groupwork, the 7th Grade science curriculum consists of 4 Units with 2-3 subunits.
This page hosts Unit 1: Chemical Reactions Roadmap with the 2 subunits within the unit.
Subunit 1: Observing Chemical Reactions
Link to this section
Below you will view and download:
🟦 Subunit Assessment Opportunities
🟦 5E Lesson Sequence
Subunit 1: Assessment Opportunities
Subunit 1 Assessment Opportunites
View and download (by making a copy)- Subunit 1 Assessments
What should my students know and be able to do?
What should I prioritize?
Note: The materials below are personal recommendations from teachers in the field.
Feel free to consider your context when deciding whether to follow these suggestions.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
View and download (by making a copy)- Subunit 1 Assessments
Subunit 1: 5E Lesson Sequence
Subunit Description
📂 Download ALL lessons at one time for Unit 1: Subunit 1 from this folder. 📂
Throughout the course of this subunit, students investigate what happens when two or more substances are combined. In their investigations, students collect and analyze data when substances are combined to determine if a physical change or a chemical reaction occurs. Students develop a model explaining that when two or more substances are combined, the result could be the formation of a mixture (physical change) or a chemical reaction (chemical change). If a chemical reaction occurs when two or more substances are combined, a new substance or substances are formed. The substance(s) formed as a result of a chemical reaction has different characteristic properties than the original substances. In their observations, students discover that chemical reactions can be accompanied by characteristic, observable changes in color, odor, and temperature.
| Lesson | Lesson Name | Teacher Document | Student Handout |
|---|---|---|---|
| 1 | Engage |
|
|
| 2 | Explore | ||
| 3 | Explain | ||
| 4 | Elaborate | 7.1 SU1 4Elaborate Teacher | |
| 5 | Evaluate | ||
📂 Download ALL lessons at one time for Unit 1: Subunit 1 from this folder. 📂
Subunit 2: Chemical Reactions at the Atomic Scale
Link to this section
Below you will view and download:
🟦 Subunit Assessment Opportunities
🟦 5E Lesson Sequence
Subunit 2: Assessment Opportunities
Subunit 2 Assessment Opportunites
View and download (by making a copy)- Subunit 2 Assessments
What should my students know and be able to do?
What should I prioritize?
Note: The materials below are personal recommendations from teachers in the field.
Feel free to consider your context when deciding whether to follow these suggestions.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
View and download (by making a copy)- Subunit 2 Assessments
Subunit 2: 5E Lesson Sequence
Subunit Description
📂 Download ALL lessons at one time for Unit 1: Subunit 2 from this folder. 📂
In this subunit, students explore what happens to atoms during a chemical reaction through a series of investigations and computer simulations. Students develop a model using the law of the conservation of matter to explain that matter is neither created nor lost during a chemical reaction, but instead that atoms are rearranged as a new substance is formed.
| Lesson | Lesson Name | Teacher Document | Student Handout |
|---|---|---|---|
| 1 | Engage |
|
|
| 2 | Explore | ||
| 3 | Explain | ||
| 4 | Elaborate | 7.1 SU2 4Elaborate Teacher | |
| 5 | Evaluate |
7.1 SU2 5Evaluate Graphic Org. HO |
|
📂 Download ALL lessons at one time for Unit 1: Subunit 2 from this folder. 📂
Unit 1: Chemical Reactions Documents
Link to this section
Below you will view and download: Unit Plan, Standards, Culminating Project Assessments and Rubrics, Common Misconceptions, Materials, Unit 0: Lift-Off Lessons and Resources.
7.1 Chemical Reactions: Overview
Overview
Through investigations, students learn to identify when a chemical reaction has occurred and model the change in molecular structure of materials showing that the number and type of atoms (mass) are conserved in a reaction. The Science and Engineering Practices of Developing and Using Models and Analyzing and Interpreting Data are used to show how scientists might model reactions and collect data to show changes. The Crosscutting Concepts of Scale, Proportion, and Quantity; Patterns; and Energy and Matter are important themes necessary for students to understand changes in atomic structure.
For the Group Culminating Project, students work together to design and construct a device that either releases or absorbs thermal energy by chemical processes. Specifically, as a group, students have an opportunity to design and construct a hot pack or cold pack. In the Individual Culminating Project, each student develops a Device Handbook explaining the science behind their device. Additionally, each student proposes at least one improvement to their device based on data gathered through testing.
7.1 Chemical Reactions: Unit Plan
Unit 1: Energy - Unit Plan
|
|
||
|
|
||
|
[Assessment Boundary: Assessment does not include valence electrons and bonding energy, discussing the ionic nature of subunits of complex structures, or a complete description of all individual atoms in a complex molecule or extended structure.]
[Assessment Boundary: Assessment is limited to analysis of the following properties: density, melting point, boiling point, solubility, flammability, and odor.]
[Assessment Boundary: Assessment does not include the use of atomic masses, balancing symbolic equations, or intermolecular forces.]
[Assessment Boundary: Assessment is limited to the criteria of amount, time, and temperature of substance in testing the device.]
|
|
|
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
||||
|
|
|
|
||
|
|
|
|||
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
||||
|
|
|
|||
|
|
||||
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|||
“Disciplinary Core Ideas, Science and Engineering Practices, and Crosscutting Concepts” are reproduced verbatim from A Framework for K-12 Science Education: Practices, Crosscutting Concepts, and Core Ideas. DOI: https://doi.org/10.17226/13165. National Research Council; Division of Behavioral and Social Sciences and Education; Board on Science Education; Committee on a Conceptual Framework for New K-12 Science Education Standards. National Academies Press, Washington, DC. This material may be reproduced for noncommercial purposes and used by other parties with this attribution. If the original material is altered in any way, the attribution must state that the material is adapted from the original. All other rights reserved.
7.1 Chemical Reactions: Standards
Chemical Reactions
Next Generation Science Standards Performance Expectations
|
|
[Assessment Boundary: Assessment does not include valence electrons and bonding energy, discussing the ionic nature of subunits of complex structures, or a complete description of all individual atoms in a complex molecule or extended structure.] |
|
|
[Assessment Boundary: Assessment is limited to analysis of the following properties: density, melting point, boiling point, solubility, flammability, and odor.] |
|
|
[Assessment Boundary: Assessment does not include the use of atomic masses, balancing symbolic equations, or intermolecular forces.] |
|
|
[Clarification Statement: Emphasis is on the design, controlling the transfer of energy to the environment, and modification of a device using factors such as type and concentration of a substance. Examples of designs could involve chemical reactions such as dissolving ammonium chloride or calcium chloride.] [Assessment Boundary: Assessment is limited to the criteria of amount, time, and temperature of substance in testing the device.] |
|
|
|
|
|
|
NGSS Lead States. 2013. Next Generation Science Standards: For States, By States. Washington, DC: The National Academies Press.
Disciplinary Core Ideas
PS1.A: Structure and Properties of Matter
- Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
- Solids may be formed from molecules, or they may be extended structures with repeating subunits (e.g., crystals).
- Each pure substance has characteristic physical and chemical properties (for any bulk quantity under given conditions) that can be used to identify it.
PS1.B: Chemical Reactions
- Substances react chemically in characteristic ways. In a chemical process, the atoms that make up the original substances are regrouped into different molecules, and these new substances have different properties from those of the reactants.
- The total number of each type of atom is conserved, and thus the mass does not change.
- Some chemical reactions release energy, others store energy.
ETS1.B: Developing Possible Solutions
- A solution needs to be tested, and then modified on the basis of the test results, in order to improve it.
- There are systematic processes for evaluating solutions with respect to how well they meet the criteria and constraints of a problem.
- Sometimes parts of different solutions can be combined to create a solution that is better than any of its predecessors.
- Models of all kinds are important for testing solutions.
ETS1.C: Optimizing the Design Solution
- Although one design may not perform the best across all tests, identifying the characteristics of the design that performed the best in each test can provide useful information for the redesign process—that is, some of the characteristics may be incorporated into the new design.
- The iterative process of testing the most promising solutions and modifying what is proposed on the basis of the test results leads to greater refinement and ultimately to an optimal solution.
Science and Engineering Practices
Developing and Using Models
Modeling in 6th–8th grade builds on Kindergarten–5th grade experiences and progresses to developing, using, and revising models to describe, test, and predict more abstract phenomena and design systems.
- Develop and use a model to describe phenomena.
- Develop a model to describe unobservable mechanisms.
- Develop a mode to generate data to test ideas about designed systems, including those representing inputs and outputs.
*Analyzing and Interpreting Data (Focal Practice)
Analyzing data in 6th–8th grade builds on Kindergarten–5th grade experiences and progresses to extending quantitative analysis to investigations, distinguishing between correlation and causation, and basic statistical techniques of data and error analysis.
- Analyze and interpret data to determine similarities and differences in findings.
Constructing Explanations and Designing Solutions
Constructing explanations and designing solutions in 6th–8th grade builds on Kindergarten–5th grade experiences and progresses to include constructing explanations and designing solutions supported by multiple sources of evidence consistent with scientific knowledge, principles, and theories.
- Undertake a design project, engaging in the design cycle, to construct and/or implement a solution that meets specific design criteria and constraints.
Crosscutting Concepts
Patterns
- Macroscopic patterns are related to the nature of microscopic and atomic-level structure.
Energy and Matter
- Matter is conserved because atoms are conserved in physical and chemical processes.
- The transfer of energy can be tracked as energy flows through a designed or natural system.
*Scale, Proportion, and Quantity (Focal Crosscutting Concept)
- Time, space, and energy phenomena can be observed at various scales using models to study systems that are too large or too small.
“Disciplinary Core Ideas, Science and Engineering Practices, and Crosscutting Concepts” are reproduced verbatim from A Framework for K-12 Science Education: Practices, Crosscutting Concepts, and Core Ideas. DOI: https://doi.org/10.17226/13165. National Research Council; Division of Behavioral and Social Sciences and Education; Board on Science Education; Committee on a Conceptual Framework for New K-12 Science Education Standards. National Academies Press, Washington, DC. This material may be reproduced for noncommercial purposes and used by other parties with this attribution. If the original material is altered in any way, the attribution must state that the material is adapted from the original. All other rights reserved.
Connections to Nature of Science
Scientific Knowledge is Based on Empirical Evidence
- Science knowledge is based upon logical and conceptual connections between evidence and explanations.
Science Models, Laws, Mechanisms, and Theories Explain Natural Phenomena
- Laws are regularities or mathematical descriptions of natural phenomena.
NGSS Lead States. 2013. Next Generation Science Standards: For States, By States. Washington, DC: The National Academies Press.
Link to Connect the 7th Grade Chemical Reactions Unit with Prior Knowledge.
7.1 Chemical Reactions: Culminating Project Assessments and Rubrics
Culminating Project Assessments and Rubrics
📂Download ALL files from 7.1 Culminating Project Assessments folder.📂
| Culminating Project File Docs |
|---|
| 7.1 Main–Culminating Projects |
| 7.1 Oral Presentation Rubric |
| 7.1 Science and Engineering Content Rubric |
| 7.1 Science and Engineering Practices Rubric |
📂Download ALL files from 7.1 Culminating Project Assessments folder.📂
7.1 Chemical Reactions: Common Misconceptions
Common Misconceptions
Lift-Off
|
|
|
|
|
|
Subunit 1: Observing Chemical Reactions
How can we tell when a chemical reaction has occurred?
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subunit 2: Chemical Reactions at the Atomic Scale
What happens to atoms during a chemical reaction?
7.1 Chemical Reactions: Materials
Materials
The Unit 1: Chemical Reactions Materials table includes all of the items needed to teach five sections of this unit in a classroom of 32 students (eight groups of four.) A detailed breakdown of how these items are used throughout the unit can be found in your Teacher Background Section at the subunit level and in each individual lesson in your Teacher Edition.
- Permanent materials have already been provided to all middle schools in the district and are expected to be reused from year to year.
- Consumable materials are replenished on an as-needed basis from year to year.
- Teacher Provided materials must be supplied by teachers each year.
Unit 1: Chemical Reactions Materials
|
|
|
|
|
|
|
|
7.1 Chemical Reactions: Subunit 0: Lift-Off Lessons
Subunit 0: Lift-Off
📂 Download ALL lessons at one time for Subunit 0: Lift-Off from this folder.📂
Lessons
| Lift-Off Lesson Documents |
|---|
| 7.1 SU0 General Groupwork Slide |
| 7.1 SU0 Liftoff Slides |
| 7.1 SU0 Liftoff Teacher |
| 7.1 SU0 Liftoff Student |
| 7.1 SU0 Liftoff Data Table HO |
📂 Download ALL lessons at one time for Subunit 0: Lift-Off from this folder.📂
7.1 Chemical Reactions: Do you want to learn more about this unit?
Do you want to learn more about this unit?
Resources
Here are some resources for Unit 7.1 Chemical Reactions:
Chemical Reactions
Middle School Chemistry “Chapter 6, Lesson 7 Multimedia.” Multimedia: Energy Changes in Chemical Reactions | Chapter 6, Lesson 7 | Middle School Chemistry. Accessed November 1, 2019. http://www.middleschoolchemistry.com/multimedia/chapter6/lesson7.
Word Generation "Middle School Science Units that Support Disciplinary Literacy," SERP, accessed November 2019. http://wordgen.serpmedia.org/science.html.
Golden Gate Bridge Highway and Transportation District
"Fog, Steel, Salt, Rust, and Paint, Golden Gate Bridge Highway & Transportation District. Accessed November 1, 2019. http://goldengate.org/exhibits/exhibitarea1e.php.
Conservation of Matter
CK–12 Brainard, Jean. “Conservation of Mass in Chemical Reactions.” CK. CK-12 Foundation, July 4, 2019. https://www.ck12.org/c/physical-science/conservation-of-mass-in-chemical-reactions/lesson/Conservation-of-Mass-in-Chemical-Reactions-MS-PS/?referrer=concept_details.
CK–12 CK-12 Foundation. “Conservation of Mass in Chemical Reactions.” CK. CK-12 Foundation, September 6, 2014. https://www.ck12.org/c/physical-science/conservation-of-mass-in-chemical-reactions/lecture/Antoine-Lavoisier-Conservation-of-Mass/?referrer=concept_details.
Other Resources Used in 7.1 Chemical Reactions
CK–12 Brainard, Jean. “Matter, Mass, and Volume.” CK. CK-12 Foundation, July 7, 2019. https://www.ck12.org/c/chemistry/matter-mass-and-volume/lesson/Matter-Mass-and-Volume-MS-PS/?referrer=concept_details.
CK–12 Brainard, Jean. “Chemical Reaction.” CK. CK-12 Foundation, July 9, 2019. https://www.ck12.org/chemistry/chemical-reaction/lesson/Chemical-Reaction-Overview-MS-PS/?referrer=concept_details.
CK–12
Brainard, Jean. “Modern Periodic Table.” CK. CK-12 Foundation, July 4, 2019. https://www.ck12.org/chemistry/modern-periodic-table/lesson/Modern-Periodic-Table-MS-PS/?referrerspecial.
Elephant Toothpaste Video “Elephant Toothpaste.” Youtube. www.chem-toddler.com. Accessed November 1, 2019.
https://www.youtube.com/watch?v=ezsur0L0L1c.
Burning Paper Video Koningsbruggen, Joey. Burning Paper. YouTube, 26 Nov. 2013, https://www.youtube.com/watch?v=TUVScBf8Znw.
SERP “Observation & Inference.” Observation & Inference • Unit [T1] SciGen SERP. Accessed November 1, 2019. https://serpmedia.org/scigen/t1.html.
SERP “Reading to Learn in Science.” SERP. Accessed November 1, 2019. https://serpmedia.org/rtls/listening.html.
CK–12 Science, Ck12. “Average Kinetic Energy.” CK. CK-12 Foundation, July 4, 2019. https://www.ck12.org/chemistry/average-kinetic-energy/lesson/Average-Kinetic-Energy-CHEM/.
View and download (by making a copy) of Resources
Note: The CC BY-NC 4.0 License does not apply to photos, images, articles, and other materials within the curriculum that have been licensed by San Francisco Unified School District and Stanford University (the Authors). These include but are not limited to photos from commercial stock photo/image agencies such as Shutterstock.com or Getty Images (iStock.com) and photos or graphics where the Authors obtained permission from organizations such as UCMP or SERP. This CC BY-NC 4.0 License also does not apply to articles that the Authors received permission to reprint [Reprinted with Permission]. You can identify such a photo, image, or licensed material by looking at the credit embedded within or associated with the content. You are allowed to reproduce the licensed material for your own personal, classroom, non-commercial use only, BUT (i) you may not modify, alter, adapt, or otherwise create any derivative work from, a licensed material and (ii) you may not distribute, transmit or disseminate a licensed material or any copy or derivative work thereof, to any third party, whether by itself, as part of a large works, or otherwise.
Note also, that throughout the student pages, there are some icons created by SFUSD and Stanford that may not have a credit line because of lack of space.
The general icons that follow were created by the San Francisco Unified School District and Stanford University and are all [CC BY-NC 4.0]:
These culminating project icons that follow were created or photographed by the San Francisco Unified School District and Stanford University and are all [CC BY-NC 4.0]:
7th Grade Science Units Link to this section
This page was last updated on July 24, 2023